produits
services
formation
boutique
contact
société
Siège international
2300 Riverchase Center
Birmingham, AL 35244
États-Unis
tél. : 888.246.8338
tél. : 205.967.7880
fax : 205.870.0304
BioHorizons Canada
21 Amber Street, Unit #6
Markham, Ontario L3R 4Z3
Canada
tél. : 866.468.8338
fax : 905.944.1894
BioHorizons Chile, S.A.
Av. Manquehue Norte 1337 Office 31
Vitacura
Santiago
Chili
tél. : +56 (2) 23619519
fax : +56 (2) 361,9521
BioHorizons Italia Srl
Via Ettore Cristoni, 88
40033 Casalecchio di Reno (BO) Italie
numéro vert +800.063.040
tél. +39.051.59.07.00
fax +39.051.57.61.06
BioHorizons Mexique
Cracovia No. 72 Torre A, Oficina: A PO-09
Col. San Ángel
CP: 01000, Ciudad de México
Álvaro Obregón, CDMX
Mexique
tel. 800.953.0498
tel. 011.52.5511638675
Spanish Office
BioHorizons Spain
Calle Oruro, 9
28016 Madrid,
Espagne
tel: +34.91.713.10.84
fax : +34.91.355.83.75
BioHorizons Camlog UK & Ireland
Reflex
Cain Road
Bracknell
Berkshire RG12 1HL
Royaume-Uni
tél. : +44 (0)1344 752560
Grafton® Matrice osseuse déminéralisée

Matrice osseuse déminéralisée Grafton
Matrice osseuse déminéralisée Grafton
La matrice osseuse déminéralisée Grafton incorpore la puissante technologie DBF (fibres osseuses déminéralisées) pour assurer une ostéoconductivité supérieure. Avec plus de 20 ans d’historique clinique, Grafton DBM vous offre la capacité de préserver la hauteur et la largeur de l’os.1 Démontré dans des études cliniques publiées, revues par les pairs, Grafton DBM sous de multiples formes offre aux cliniciens des options pour les applications de greffe osseuse.2
- Indiquée pour remplir des cavités osseuses, augmenter le volume d’une greffe osseuse et comme substitut de greffe osseuse3
- Disponible dans de multiples formes pour offrir les caractéristiques de manipulation souhaitées pour de multiples indications cliniques

Layout Style for Content Bar
applications concernées4
- greffe osseuse alvéolaire après extraction
- augmentation de crête et sinus
- défauts kystiques
- augmentation cranio-facial
- remplissage de défauts parodontaux

cas Grafton DBM
augmentation de la crête
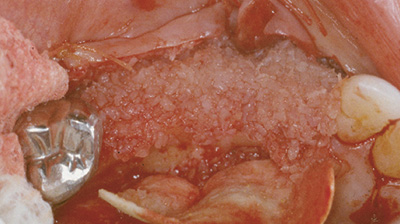
greffe composite de Grafton DBM et FDBA
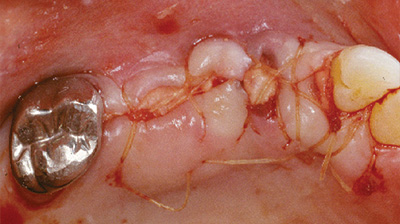
AlloDerm GBR utilisé en tant que membrane protectrice
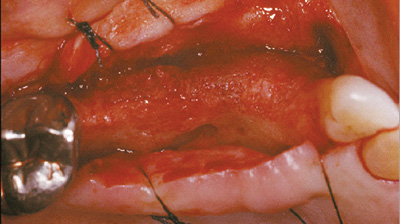
largeur régénérée pour permettre une pose de l’implant dentaire
Layout Style for Content Bar
documentation
Default Position for Info
| Catalogue produits |
| Catalogue produits internationaux |
| Examen clinique |
| Fiche comparative des tissus durs |
Select Additional Info Type
- Analyse histologique de sites implantaires après greffe de matrices osseuses déminéralisées en Putty ou en feuilles. Callan DP, Salked, SL, Scarborough, NL Implant Dent. 2009;9(1):36-42.
- Les différentes formes de la matrice osseuse déminéralisée Grafton et la matrice osseuse déminéralisée Grafton Plus en pâte ont reçu l'agrément FDA 510(k) afin d'être utilisées pour remplir des cavités osseuses, pour augmenter le volume d'une greffe osseuse ou comme substitut de greffe osseuse.
- Déclarations des modes d’emploi et des résumés sur l’innocuité et l'efficacité d’utilisation de la FDA 510(k) K051188 et K051195 pour remplir des cavités osseuses, augmenter le volume d’une greffe osseuse et comme substitut de greffe osseuse.
- Veuillez vous référer au mode d'emploi du fabricant disponible dans la notice d'accompagnement.








